Blog
SL Controls US Expansion Through Existing International Corporate Client
Over the years, SL Controls has completed various projects outside Ireland for clients in the pharmaceutical and medical device industries. However, we took this international growth a stage further in 2019 by opening a new office in Florida, USA. We did this through an existing international client we have a strong and long-standing relationship with.
Patrick Potter is Florida Operations Lead for SL Controls, so he is the person who led the expansion into the US.
Patrick said: “The first client we started working with in the US has a sister plant in Ireland that we have completed projects for over a number of years. Therefore, we have a detailed knowledge of the products they are manufacturing as well as the machines, equipment, and platforms they use.
“It made sense to transfer that knowledge to the plant in the US, delivering the same productivity, efficiency, and OEE improvements to the US operation that we have delivered – and continue to deliver – in Ireland.”
Building on Our Reputation
Delivering according to client requirements and expectations is central to everything we do. Darragh McMorrow, Commercial Director at SL Controls, says expansion into the US is a significant milestone in the company’s growth plans.
“Having an established relationship with this client and then being trusted to deliver similar projects at the US-based sister manufacturing facility of the Irish plant is something we are very proud of.
“Technology in our sector moves incredibly fast as new and increasingly innovative Industry 4.0 and Smart Factory-related technologies come on-stream.
“The regulatory requirements that our clients must follow also mean new and creative solutions need to be developed.
“In Ireland, we are well-established as the leading provider in the delivery of these solutions. We’re also now well on our way to building a similar reputation in the US.”
The Practicalities of Expanding in the US
As he was the lead in the process, Patrick now has key first-hand experience of establishing operations in another country. Before he took on the role in the US, he was a Project Manager working from one of SL Control’s Irish offices.
Patrick said: “I’ve worked in the UK before, so I have some experience of working abroad, but this was a bit different. I wasn’t moving to an established office in the US – I was moving to start the office.
“It was a brand-new venture and, although SL Controls has done work in the US before, I had never been to the US for SL Controls. However, it was an opportunity to see other parts of the world. So, I was very interested, and so was my wife. In the end, it was an easy decision to make the move.”
Patrick spent five weeks in the US in October 2018 to get a feel for working in a different country. The plan was then to return in January although the process for obtaining a suitable visa turned out to be “painfully slow”.
Patrick has now been in the US since July, setting up home – and the new SL Controls office – in Jacksonville, Florida.
Initial Challenges But Significant Opportunities As Well
“There are challenges,” said Patrick. “I know the systems and technologies very well because of our work on the client’s sister plant in Ireland, but there are little things you have to start again with from scratch, such as establishing working relationships with key personnel in a brand new working environment.
“But, getting to know the right people was only a minor item at the start. In a short space of time, I have developed excellent working relationships with my new colleagues which have been a major help in allowing me to settle into my new environment so quickly. I also now live in the Florida heat and sunshine, which is a big plus.
“It also looks to me that there is a serious opportunity here in the US too. It’s the size and scale of this country and the number of clients and manufacturing plants that exist. I mean, Florida is bigger than Ireland and it is only one state.
“Also, automation is a growing force here in the US. Automating manufacturing and business processes is a key priority for companies, and SL Controls has a proven track record of delivering projects in this area.
“Looking to the future, the potential for SL Controls in the US, with our new office in Florida now established, is highly promising.”

Video: Learn More About SL Controls, What We Do, and What’s Important to Us
At SL Controls, we deliver bespoke software solutions to manufacturers in the medical device and pharmaceutical industries. We work with a wide range of clients in these industries, including many of the largest.
Our success is built on the quality and commitment of our people. Watch our video to find out more.

Using Outsourcing to Achieve Digital Transformation Success in the Pharmaceuticals and Medical Device Industries
Digital transformation involves more than digitising various aspects and processes of your business. It’s not about adding cool new tech or feature-packed software programmes either.
Instead, digital transformation is about digitalising your business, i.e. transforming your production facility into a digital business. This means interconnecting processes within the production line and in other parts of the business to add value to customers, improve productivity, make efficiency savings, enhance product quality, etc.
It’s an evolutionary process, but how can you make sure you implement digital transformation projects successfully and in a way that delivers an effective return on investment?
The Challenge of Digital Transformation in Pharma and MedTech
The above is often a challenging question to answer in the pharmaceuticals and medical device industries. After all, you have limited resources that are already busy with existing tasks and operations. How do you undertake the necessary but often complex process of digital transformation on top of this existing work?
Also, your existing resources may not include all the expertise and experience required to deliver a digital transformation project.
Outsourcing is an essential part of the solution.
Outsourcing Digital Transformation
To clarify, this isn’t just about bringing in a third-party solution provider to deliver on a project that involves non-core aspects of your business.
Instead, outsourcing digital transformation involves developing relationships with strategic partners that offer resources, expertise, experience, and knowledge to ensure you achieve digital transformation success over the long-term.
7 Benefits of Outsourcing Digital Transformation
1. Additional Resources
Outsourcing digital transformation will ensure sufficient resources are available to deliver on the project’s objectives. Furthermore, the responsibility for ensuring those resources are in place moves to the outsourced provider, allowing you to get on with running your business.
2. Access to Expertise
You will also get access to highly valuable expertise and experience when you outsource digital transformation. Providing you select the right team, there will be engineers working on your project who have delivered similar solutions in the past. The right team will also have experience in the health sciences sector.
3. Full Range of Capabilities
The full range of capabilities you will have access to by outsourcing cannot be understated. After all, you may be able to resource-up in-house for the main skills required to complete a particular project, but you are unlikely to get all the skills you need.
When you outsource, you have immediate access to people with the skills, training, knowledge, and experience required to progress the project and implement it successfully.
4. Reduce Risk
When you undertake a digital transformation project in-house, you are responsible for all the risks involved. This fact is even more pertinent if the project involves technologies or solutions your team is not familiar with.
By outsourcing, you share the risks, plus the outsourced team will have experience of similar projects and the challenges those projects face.
So, not only can you share the risk, but you will also reduce risks associated with the project.
5. Faster Project Completion Times
An experienced outsourced team will have a knowledgebase in addition to experience, both of which can be used to ensure your project is delivered as efficiently as possible. This cuts project completion times and minimises delays.
6. Flexibility
You have more flexibility when you outsource digital transformation projects compared to completing them in-house. More specifically, with an outsourced partner, you can proceed with digital transformation in stages according to the requirements of your business, including your budget.
7. Scalability
Outsourcing also makes it easier to scale up or down the pace of digital transformation and your facility’s progress.
Find Out More
At SL Controls, we partner with many of the biggest pharmaceutical and medical device companies in the world to deliver a wide range of digital transformation projects. Find out more today.

The Most Important Smart Factory Trends for Pharmaceutical and MedTech Manufacturers in 2020
The development of the smart factory is a key priority for manufacturers in all industries and sectors. It’s part of the digital transformation of business, and it’s essential to remain competitive as well as to meet the needs of consumers and end-users as well as B2B customers and regulators.
This all comes under the umbrella of Industry 4.0, the moniker for the revolution the manufacturing sector is currently going through.
Like the industrial revolutions before it, however, Industry 4.0 will take many years and decades to develop as new technologies and processes are created, and as companies, regulators, and consumers evolve.
So, what about the immediate future of the Smart Factory? What are the digital transformation trends that you need to be aware of for 2020?
In particular, what are the important smart factory trends that you should be considering now if you are in the pharmaceutical or MedTech industries?
Trend 1 – Big Data Analysis
Much of what is possible now and will be possible in the future with the Smart Factory is critically dependant on how we manage data, i.e. our ability to capture, transmit, store, analyse, present, and use data in a way that enhances manufacturing operations and the wider business.
With the ability to collect and store data, it’s not surprising that the volume of data is growing considerably. Making sense of and then putting to practical use this larger volume of data becomes the next challenge.
Big data analysis is the solution, and it will become increasingly important in 2020 and beyond.
Trend 2 – Predictive Analytics
One crucially important way of using big data is predictive analytics. With predictive analytics, you can anticipate problems, mitigate risks, take advantage of opportunities, and ensure resources are targeted in the right areas.
One way that pharmaceutical and medical device manufacturers are already using predictive analytics is in the area of machine maintenance.
Using technologies like digital twins, it’s possible to predict the impact that a variable will have on a particular piece of equipment, a production line, or on a process.
Information from sensors can also be analysed to predict when failures are likely to happen, enabling the scheduling of maintenance before the failure occurs.
Machine maintenance is only one area where predictive analytics is going to become increasingly important, however.
Manufacturers will also be able to improve their predictions on how markets will change, how risks will impact future performance, the impact of product or business expansion, etc.
Trend 3 – End-Customer Focused Business Models
The average consumer is changing in a number of ways, including placing increasing demands on manufacturers to offer personalised products.
This is even more apparent in the life sciences industries where there are considerable benefits to supplying patients with personalised medical devices and even drug products.
Manufacturers in the medical device and pharmaceutical industries are increasingly adapting their business models and production operations to meet these demands, not just for patients but also for health care professionals and organisations. In other words, they are moving from being solely B2B operations to having significant B2C operational components.
Examples of the technologies and processes that are becoming increasing in-play are manufacturing batch sizes of one and mass customisation.
Trend 4 – Increasing Focus on the Internet of Things (IoT)
When you hear talk in the media about the IoT, the examples cited are often for things like fridges being able to order milk and ovens you can control from your smartphone.
These applications of IoT technologies might be beneficial, but there are also more powerful applications that manufacturers can benefit from right now.
Just to recap, IoT technologies enable the connectivity of anything, anywhere.
So, manufacturers can add IoT technologies to the products they produce to get real-world data on how customers actually use them, rather than basing their knowledge on how they think customers use their products. This information can then be fed into the marketing and product development process as well as into areas like customer service and quality assurance.
IoT technologies can also be used to improve the supply chain, ensure complete product traceability, enhance customer service, and more.
Trend 5 – Further Implementation and Integration of Existing Smart Factory Technologies
Many manufacturing operations in the pharmaceutical and medical device industries already use technologies that can, depending on how they are applied, come under the Smart Factory umbrella. Examples include:
- Collaborative Robots (Cobots)
- Autonomous vehicles
- AR (Augmented Reality) and VR (Virtual Reality)
- Digital Printing
- Advanced Process Control (e.g. PAT – Process Analytical Technology)
Another trend for 2020 and beyond that we expect to see is the increasing use of these types of technologies to find additional value throughout the supply chain.
Moving Forward
The lights-out factory has become a jargon term laden with both positive and negative connotations. For most manufacturers in the medical device and pharmaceutical manufacturing industries, however, the lights-out factory is not a short-term or even medium-term consideration.
However, digital transformation – or the digitalisation of businesses – as well as the Smart Factory are considerations for today to improve product and service offerings or even to revolutionise and disrupt business models. Moving forward in these areas is essential for commercial survival.

The Benefits of Third-Party Contractor Solutions for Manufacturers with Engineering Skills Gaps
Skills gaps can arise in your organisation for several reasons. You might, for example, have a project you want to complete to improve productivity, enhance OEE, or give you better management oversight.
Regulatory changes, temporary staff shortages, issues that need to be resolved, new product introductions, production line relocations, and changes in business strategy are other common causes for the creation of skills gaps in your manufacturing operations.
The solutions available to fill those skills gaps include:
- Hiring new employees
- Bringing in additional independent contractors
- Working with a third-party that specialises in providing professional placement services that cover the skills you need
All three of the above solutions have merit and are suitable in a range of situations.
Increasingly, however, companies are turning to the third solution as their preferred option. This particularly applies to manufacturers in highly regulated, high-volume, and/or high precision industries. Good examples include pharmaceuticals and medical device manufacturing.
Rapidly Advancing Technologies, Enhanced Customer Expectations, and Changing Regulatory Landscapes
In the past, engineers and other specialists required by manufacturing operations could focus on specific areas of expertise. Qualified mechanical engineers worked as mechanical engineers, for example, while programmers worked as programmers and electrical engineers worked as electrical engineers.
The world has changed, however, with those changes continuing at a considerable pace. The factors driving these changes include:
- The introduction of new technologies
- The move to Smart Factory solutions as part of the push into Industry 4.0
- Increased levels of competitiveness
- Enhanced customer expectations in relation to everything from aftersales support to individual product customisation
- New regulatory requirements
The result is that engineers now need to be multi-skilled and multi-disciplined. At SL Controls, one newly developing branch of engineering we believe typifies this is Smart Manufacturing. Engineers working on Smart Manufacturing projects must have programming, mechanical, electrical, controls, and validation skills.
From your perspective, you not only need to fill skills gaps, but you also need to get people with a sufficient range of skills. Plus, you need a solution that makes business sense and delivers a tangible return on investment.
This is what you get when you choose professional placement services from a specialist third-party provider over the other solutions that are available.
Benefits of Third-Party Professional Placement Solutions for Manufacturers
- You’ll get access to a wider range of skills than you get when you employ a single person or hire an individual contractor.
- You will benefit from the collective knowledge and experience of the company you choose to work with. The best professional placement solution providers have worked on hundreds of projects, including projects similar to yours. You will benefit from that full breadth of in-house learning and collective expertise.
- Choosing the right provider will ensure you get people working in your production facility and on your projects who have direct experience in your industry. Given the unique nature of industries like pharmaceuticals and medical devices, this is crucially important.
- You can get the additional resources you need into your business faster.
- You’ll be up and running with the expertise your business needs from day one. After all, you are buying ready-to-go skills.
- The relationship will be both project-focused and results orientated.
- You only pay for what you need while having maximum flexibility to set expectations, requirements, parameters, and more.
- There are no additional costs commonly associated with hiring a new employee such as recruitment and training costs.
Staying Competitive
Your production facility – and wider business operations – can’t stand still.
Digitalisation is no longer a buzzword – it’s a necessity. Regulations are not going to get laxer. Customers are not going to start accepting lower standards.
To stay competitive, you need to constantly improve, working to maximise OEE, find new business opportunities, build closer relationships with your customers, automate more of your production processes, integrate your operations, drive efficiency savings, make productivity gains, and increase profitability.
Working with an engineering solutions provider that specialises in professional placement services will give you the skills you need to achieve all the above and more.

Digital Transformation in Pharmaceutical and Medical Device Manufacturing Companies
Digital transformation is now an everyday reality in pharmaceutical and medical device manufacturing companies. New and developing technologies create opportunities for digital transformation to take place, but the main driver is strategic planning and decision-making.
In other words, senior executives in the pharmaceutical and medical device industries are increasingly prioritising digital transformation.
There are good reasons for this. In manufacturing facilities around the world, for example, digital transformation is improving OEE, enhancing productivity, driving efficiency savings, and delivering return on investment.
The Path to Digital Transformation
New technologies combined with a business strategy that embraces digitalisation creates opportunities in pharma and MedTech businesses to change, improve, and identify new opportunities in a range of different business areas. This includes business management and culture, product development, supply chain management, marketing, customer service, regulatory compliance, and more.
The technologies being used include automation, AI (artificial intelligence), machine learning, data analytics, blockchain, and more.
The Benefits of Digital Transformation
A substantial portion of companies in the health sector, including pharmaceutical and medical device manufacturers, has started the process of digital transformation. For some, though, this progress amounts to dipping a toe in the water.
The benefits and opportunities that digital transformation presents, however, are too great to ignore.
Those benefits include:
- Improving efficiency and productivity – crucially, these benefits go beyond the traditional silos that exist in the manufacturing industry. The real benefits lie in efficient and productive business units that are truly integrated, i.e. production lines integrated with supply chain management, finance, customer service, marketing, and product development.
- Identifying new business opportunities – for example, through the use of data analytics to develop new products, by using data modelling to prove the long-term cost savings of preventative treatments, increasing consumer/patient and prescriber choice through mass customisation-enabled manufacturing operations, and more.
- Improving patient outcomes – by developing new products, establishing more efficient and robust supply chains, reducing manufacturing costs, improving product quality, etc.
- Building better relationships with patients and consumers – with fully customisable products, new technologies like wearable tech that improves diagnoses and treatment decisions, the smooth availability of supply, improved product quality, faster and more accurate resolution of problems, etc.
- Building better relationships with prescribers – through helping them improve the level of care they offer by, for example, giving them more effective products, more innovative and efficient supply processes, greater access to information and support, etc
Improving Manufacturing
Manufacturing is one of the most significant areas that pharmaceutical and medical device companies are seeing improvements delivered by digital transformation.
Fully automating repetitive manufacturing processes that currently require human intervention is one example of a practical and realistic application of technologies that come under the digital transformation umbrella. It’s also an example that delivers rapid return on investment.
Other practical and realistic digital transformation applications include:
- Predictive maintenance – using sensor, communication, cloud computing, digital twin, and data analytics technologies to substantially reduce unplanned equipment downtime and improve OEE
- Product traceability – digitalising the product traceability process with serialisation solutions not only improves accuracy, but makes it possible to access detailed product data in seconds
- Management oversight – making it possible for senior engineers and managers to oversee manufacturing operations from anywhere in the world using cloud computing, communication, and remote access and control technologies.
- Equipment systems integration – using software programmes to fully integrate machines, platforms, systems, and equipment regardless of vendor, physical location, or business unit.
- Server infrastructure management – using cloud technologies to significantly reduce the financial and resource burden of on-site server infrastructure management and support.
- Technical and engineering support – technologies like augmented reality, mixed reality, video conferencing, and remote access reduce the cost of engineering and technical support while also improving the efficiency of that support.
Conclusion
The future of the pharmaceutical and medical device manufacturing industries is changing as a result of digital transformation. From the perspective of your organisation, you don’t need a revolutionary approach. Constantly moving forward, improving, and migrating your business to a digital model is, however, essential.

Video: Professional Placement Services for the Pharmaceutical and Medical Device Manufacturing Industries
At SL Controls, we can help if you need to boost your engineering team. Our professional placement and consultation services will give you the engineering expertise you need either on a short-term or long-term basis. We specialise in providing these services to the pharmaceutical and medical device manufacturing industries.
Watch our video to find out more.

Video: Find Out More About Our Equipment Systems Integration Solutions
At SL Controls, we offer a comprehensive range of Equipment Systems Integration solutions for manufacturers in the Pharmaceutical and Medical Device industries. Our solutions enhance OEE and productivity as well as being fully bespoke. They will also deliver a measureable return on investment.
Watch our new video to find out more.

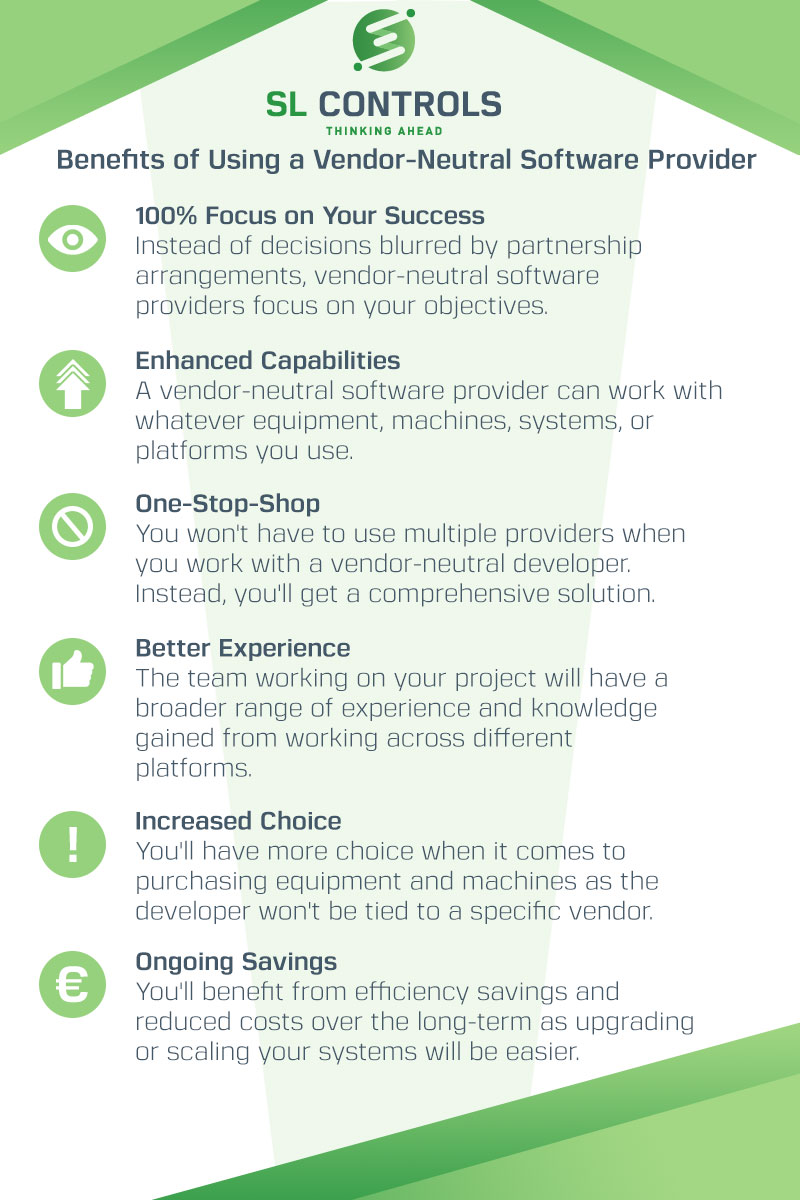
Infographic – Benefits of Using a Vendor-Neutral Solution Provider
At SL Controls, we are a vendor-neutral solution provider to the pharmaceutical and medical device manufacturing industries. We can work with equipment, platforms, software, and systems from any vendor.
In addition, we are not tied to specific vendors when we recommend equipment or platforms to purchase.
The benefits of using a vendor-neutral software provider like SL Controls includes:
- 100% focus on your success
- Enhanced capabilities
- One-stop-shop
- Better experience
- Increased choice
- Ongoing savings
Our infographic explains more.
« Previous 1 … 9 10 11 12 13 … 18 Next »




