What is ALCOA+ and Why Is It Important to Validation and Data Integrity
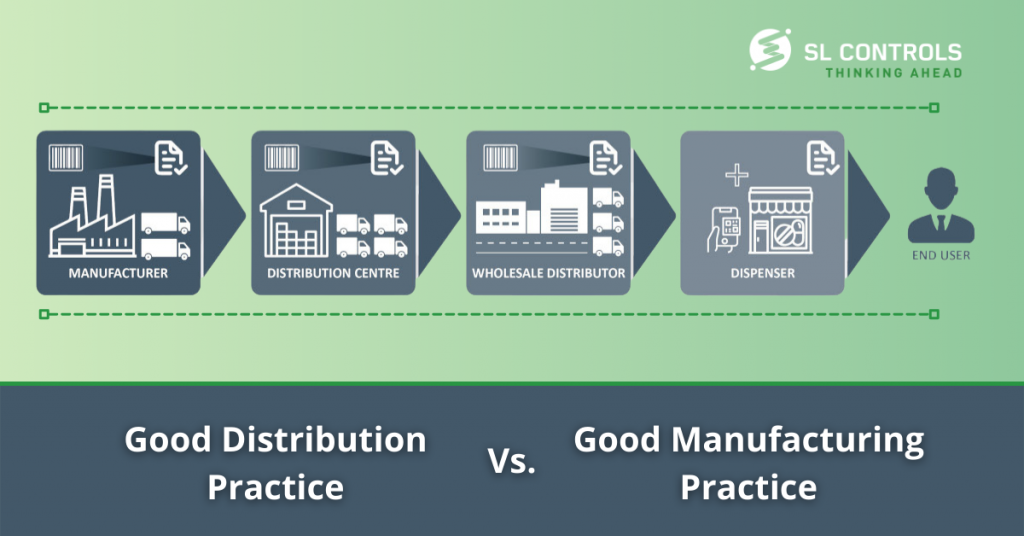
ALCOA+ is a set of principles that ensures data integrity in the life sciences sector. It was introduced by, and is still used by, the FDA – the US Food and Drug Administration. It has relevance in a range of areas, particularly in relation to pharmaceutical research, manufacturing, testing, and the supply chain. As well …
What is ALCOA+ and Why Is It Important to Validation and Data Integrity Read More »