Where Do We Stand with the New EU MDR, Additive Manufacturing, and Customised Medical Devices?
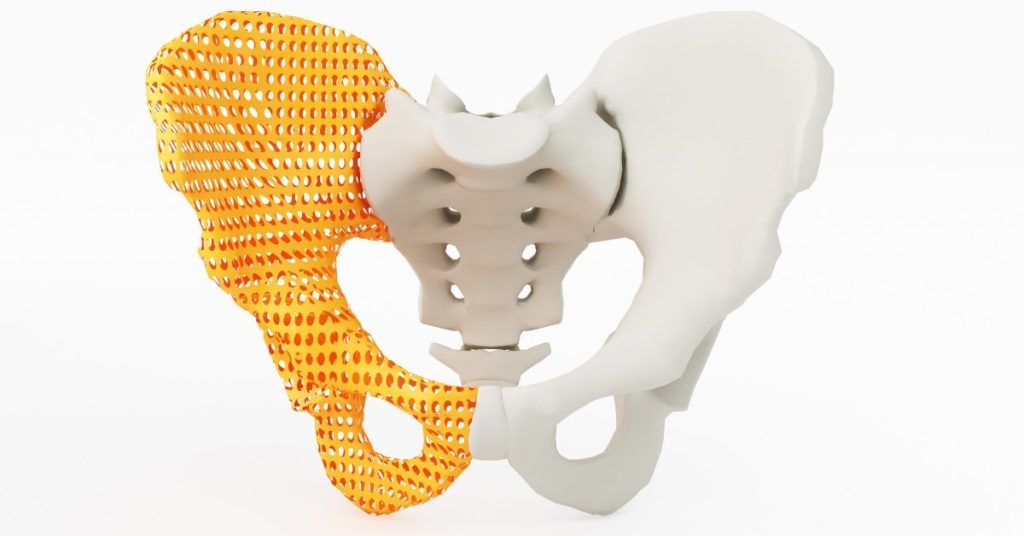
One of the growing trends in the medical device industry is customised products for individual patients. An example is an implant designed specifically for an individual patient. A range of technologies make products like this possible, including new additive manufacturing technologies. Furthermore, customised medical device products offer significant benefits to patients. There are benefits to …