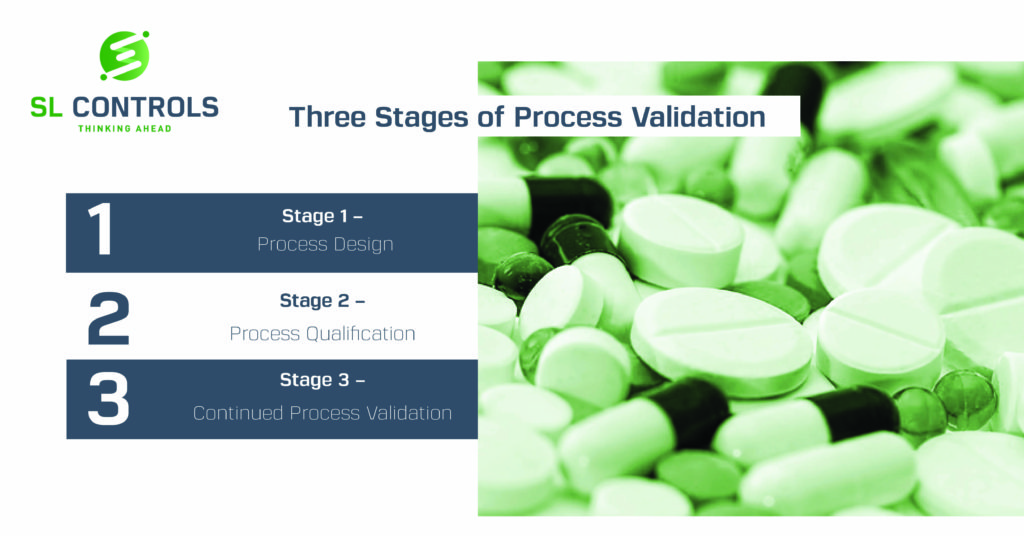
Digital Validation and Why It’s Important to Pharmaceutical and MedTech Manufacturers
Modern technologies make it possible to create digital simulations, digital twins, and virtual 3D models of products, parts, manufacturing processes, machine processes, systems, and more. Engineers can then use these simulations and models to facilitate the design process, improve quality, identify errors, and improve performance. They can do this by testing multiple design variations in …
Digital Validation and Why It’s Important to Pharmaceutical and MedTech Manufacturers Read More »