Good Distribution Practice (GDP) is a quality system for warehouses and distribution centres that process medicines. Historically, GDP only covered the movement of finished products from manufacturing sites to wholesalers, retailers, and patients. These parts of the supply chain are still covered by GDP, but the guidelines are now applicable throughout the whole supply chain, from raw materials to the finished product and everything in between.
The EU added this enhanced GDP requirement to its guidelines in 2013 because of increased globalisation, the sensitivity of certain therapeutic drug molecules, and the stricter controls that are now required to maintain the quality and integrity of medicinal products.
Failure to adhere to the guidelines at any point in the supply chain, including all intermediate points of storage and transport, can have a serious impact on the quality of the product.
Is Good Distribution Practice Essential?
For companies involved in sourcing, storing, and transporting APIs and other ingredients for pharmaceutical manufacturing, the adoption of GDP is a crucial tool to ensure patient safety, product integrity, and product availability. It is also essential for similar reasons for companies involved in the distribution of finished products to the end-user.
Here is how EU regulators define GDP in the guidelines:
“These Guidelines lay down appropriate tools to assist wholesale distributors in conducting their activities and to prevent falsified medicines from entering the legal supply chain. Compliance with these Guidelines will ensure control of the distribution chain and consequently maintain the quality and the integrity of medicinal products.”
The guidance also says that wholesale distributors must comply with GDP to obtain wholesale distribution authorisation. Wholesale distribution authorisation is required for the wholesale distribution of medicinal products in the EU.
Why Thermal Compliance is Relevant to GDP
Product deterioration is one of the main challenges that pharmaceutical companies face when ensuring patient safety and product quality. As deterioration is significantly impacted by temperature and humidity, the following two areas need to be addressed to maintain products in an optimal condition during storage and distribution:
- Ensuring storage conditions follow product labelling requirements (for example, not storing above 25°C)
- Providing documented evidence that demonstrates consistent adherence to the storage requirements listed on product labels
By following GDP, the above can be achieved through:
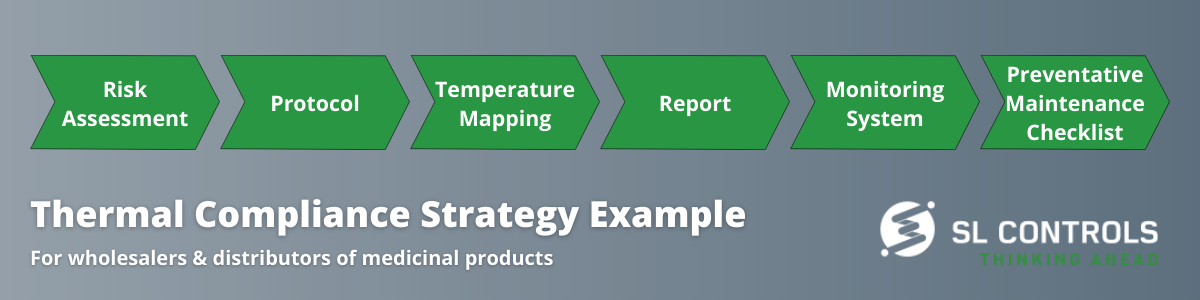
- Documented temperature mapping studies (empty/full/seasonal/challenge conditions)
- Documentation that temperature monitoring placement is determined by a risk assessment of high/low thermal risks as identified through temperature mapping studies
- Continuous temperature monitoring system with timely alerts of temperature deviations
- Calibration of temperature monitoring systems
- Ensuring compliance with standards on temperature/humidity mapping and monitoring, shipper route qualifications, calibration, and validation
GDP Compliance Situations that Companies May Face
Pre-Audit Assessment
Businesses should always be ready for inspection. However, a letter from regulators announcing a date for inspection can cause high levels of stress and disruption due to a lack of readiness. To ensure your organisation is better prepared, you should conduct a pre-audit assessment to understand your level of conformance with EU guidelines, including GDP guidelines.
If You Have an Existing Wholesale Licence
Audits are part of holding a licence to store and distribute medicines in the EU. You might face a situation where an audit from a regulatory authority, such as the HPRA in Ireland, raises issues with your level of compliance.
A common example of such an issue is the placement of continuous monitoring probes. In this example, a thermal profile should be created through seasonal temperature mapping, with the location of monitoring probes determined by that profile.
New Application for Wholesale Licence
If you are preparing to make a wholesale licence application to a regulatory authority, you will need to ensure compliance with GDP guidelines. This includes guidance on thermal compliance. For example, you will need to:
- Assess the capability of your facility to store medicinal products within the required range of temperatures
- Create a thermal compliance strategy
- Establish an effective thermal monitoring system
- Validate the thermal monitoring system and compliance strategy
- Ensure the ongoing integrity of the system through annual calibration and a preventative maintenance approach
Here is an example of a typical strategy for ensuring compliance:
Product Quality, Patient Safety, and Compliance
GDP is rightly looked at as a compliance issue as there are guidelines and regulations that need to be followed. However, it is also important to remember that compliance is only one of the benefits of GDP for your wholesale or distribution operation.
Adhering to GDP helps you maintain product quality standards, enhancing the service you offer to clients. It also helps you meet your responsibilities in relation to patient safety.
In other words, adhering to GDP has commercial as well as regulatory benefits.





