Validation Services
SL Controls provides a full range of validation services for high-volume and highly regulated industries including pharmaceutical and medical device manufacturers. We can assess your existing validation process, or we can implement a new validation solution to meet your specific requirements. From initial concept to project delivery and ongoing support, SL Controls is the industry expert in Validation.
Speak to a member of our Validation team today to find more about how we can help your production process. Email [email protected] or complete the form and we’ll get back to you.
Validation Services For Entire Product Lifecycles
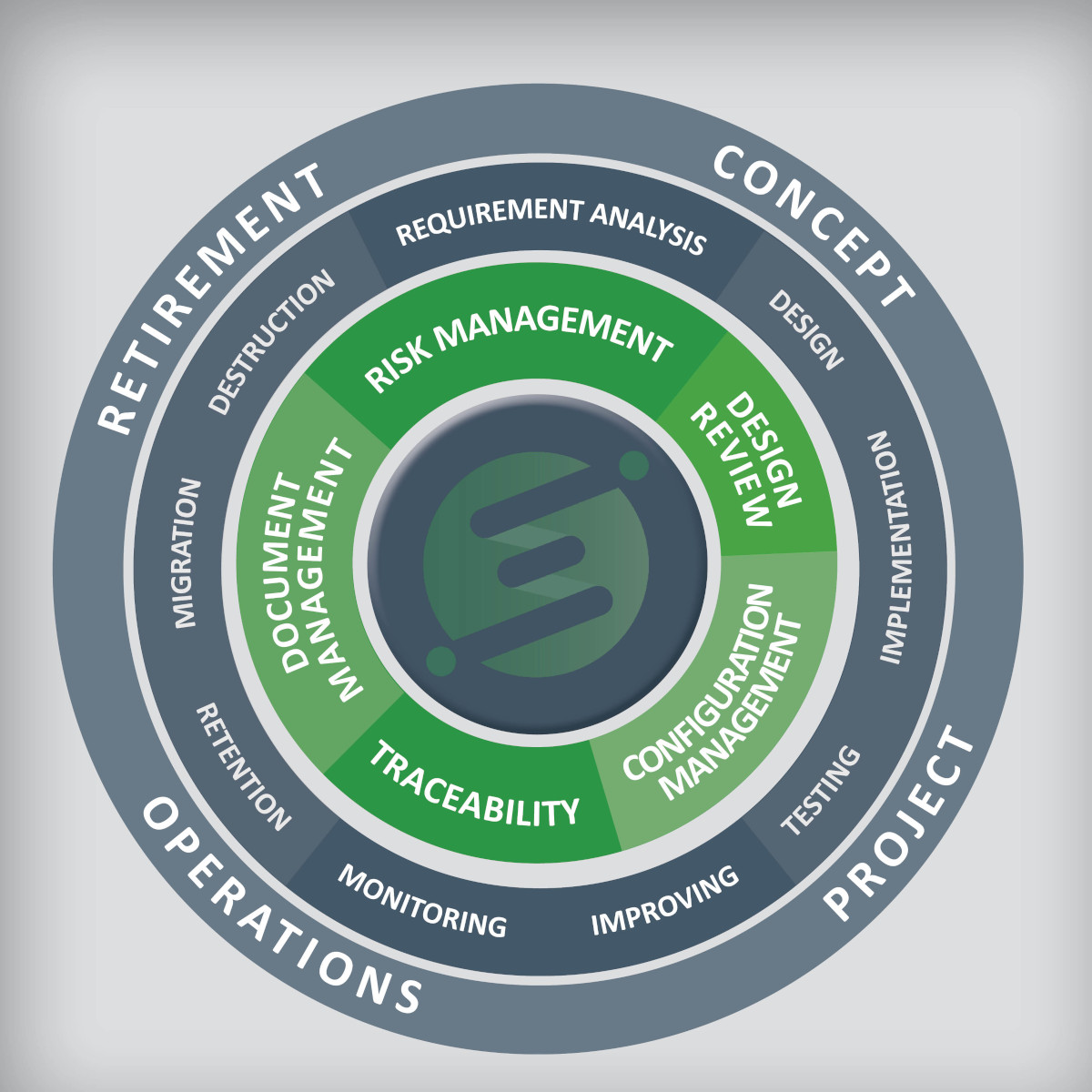
At SL Controls, we offer end-to-end validation services. This includes:
- Concept to project to operation to retirement.
- Requirement analysis, implementation, testing, improving, monitoring, retention, migration, and destruction.
- Risk management, design reviews, change and configuration management, traceability solutions, and document management.

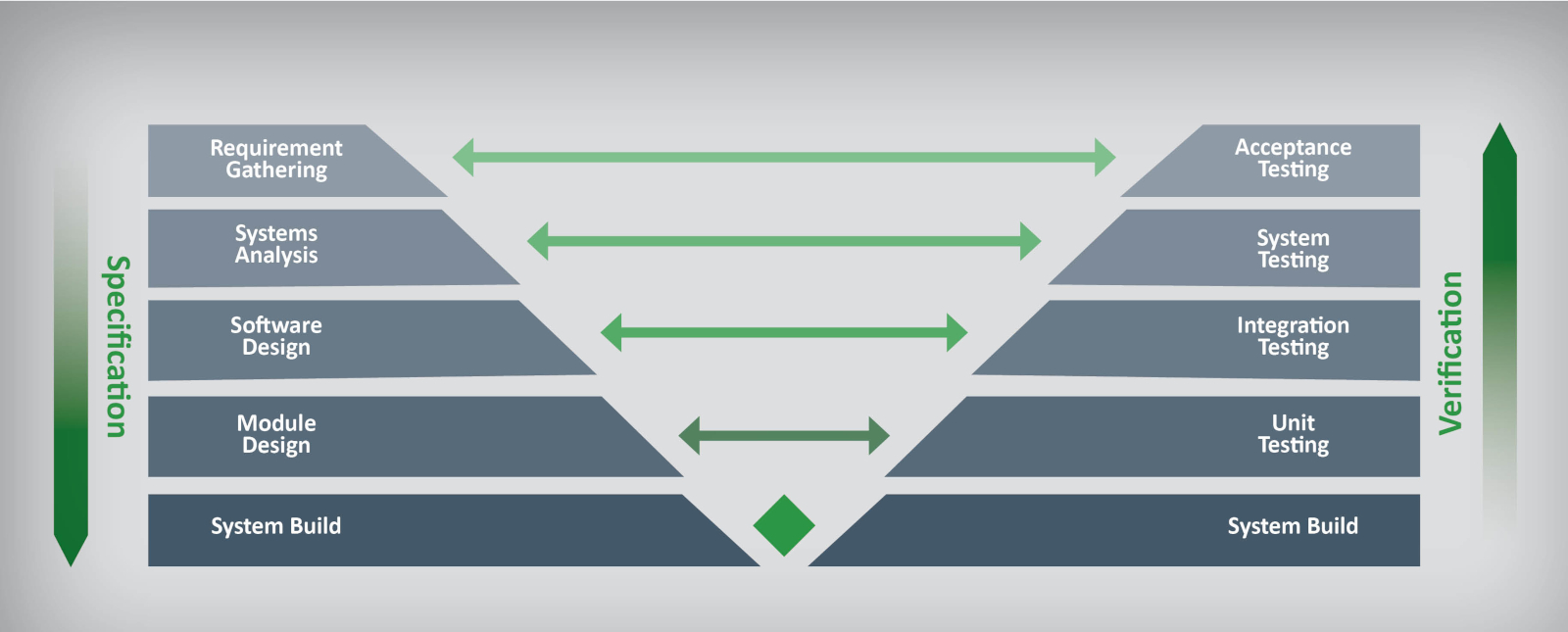
Our software validation expertise includes:
- Planning – design and development planning.
- Verification – design verification.
- Testing – test plan preparation, testing, and acceptance.
- Traceability – linking requirements through the validation process.
- Configuration management – to ensure proper documentation and to control development activities running in parallel.
- Quality – ensuring software meets quality controls.
- Risk management – documenting compliance and business risks and developing mitigation strategies.
- CSA – using the more flexible Computer Software Assurance risk-based approach instead of traditional CSV.

At SL Controls, we have extensive experience using Computer Software Assurance (CSA) methods to validate non-product software in manufacturing environments. Examples include your ERP, QMS, MES, and PLM. When using CSA, we take an approach that is focused on the end-user of your products while also being value and quality-driven.
CSA is a risk-based approach to validation that is more efficient as validation cycles are shorter and less expensive. With the implementation of CSA, you can expect savings in both costs and time that are as high as 50 percent.
Our expertise includes the development of risk management processes, assisting with risk categorisation, and determining when to use scripted, unscripted, and ad hoc testing of software systems.
Get in Touch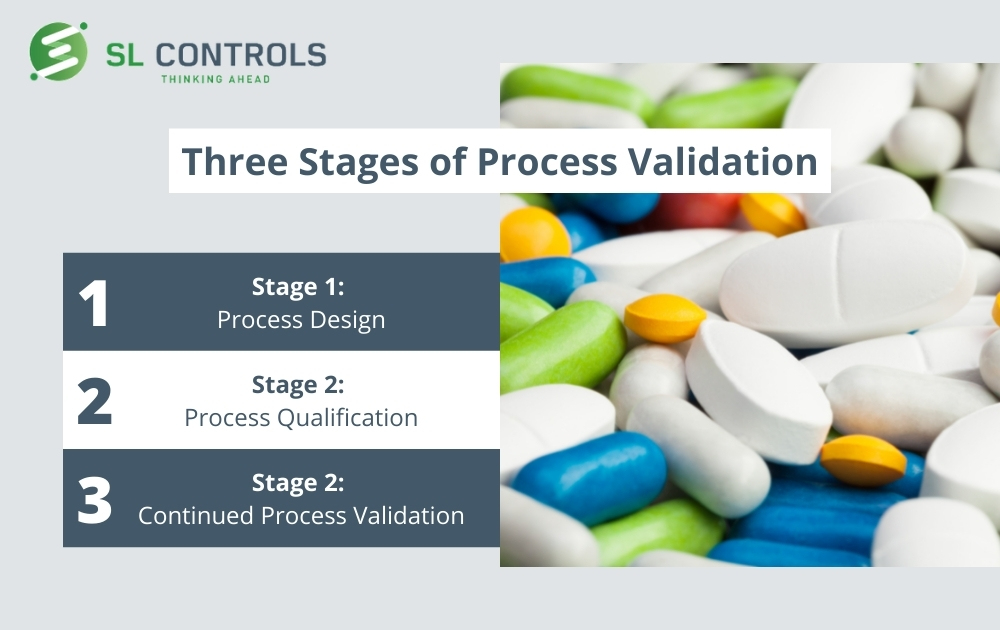
Our process validation solutions will ensure your manufacturing facility remains in compliance with FDA and European regulators. We have extensive experience and in-depth knowledge of GMP, including modern risk-based techniques and methods. We can help with all three stages of process validation, including:
- Process design – designing a process that ensures the products you manufacture consistently meet quality requirements.
- Process qualification – evaluating the process design, including process performance qualification (PPQ) and qualifying/evaluating your facility’s design, utilities, and equipment.
- Continued or ongoing process verification – to ensure your commercial manufacturing operations remain in a state of control.

At SL Controls, we offer vendor-neutral equipment validation solutions. Equipment validation is an essential factor in the production of products that consistently meet quality standards. Our expertise includes equipment validation in your project’s initial design stages, installation qualification, the qualification of operational equipment, executing test protocols, performance qualification, and process qualification.
We’ll make sure your equipment is qualified to cGMP standards to ensure compliance and to meet your quality standards. We’ll also make sure your solution produces the reports and documentation that you need.
Get in Touch
At SL Controls, we provide a full range of test method validation solutions to ensure the suitability of your pharmaceutical or medical device test methods. We have extensive knowledge of the compliance requirements of European regulators and the FDA, as well as various test methods. The latter includes content uniformity assays, friability, disintegration, packaging integrity, weight conformity, and visual identification testing methods.
Our services include:
- Establishing performance characteristics
- Identifying limitations of the method
- Identifying influences

- Specialised validation department.
- Experienced validation engineers.
- Solutions from concept and strategy through to delivery.
- Extensive experience with over 200,000 validation hours completed.
- Full end-to-end validation services.
- International projects delivered in the UK, US, Europe, and Asia.

Download our Validation case studies to find out more about how our customers have benefited from our validation services.
Get in TouchSpeak to a member of our Validation team today to find more about how we can help your production process. Email [email protected] or complete the form and we’ll get back to you.




