The FDA (US Food and Drug Administration) is due to release guidance on Computer Software Assurance. That guidance aims to put critical thinking at the centre of Computer System Validation (CSV) processes.
In our latest infographic, we highlight the main differences between traditional CSV and the new Computer Software Assurace (CSA) approach.
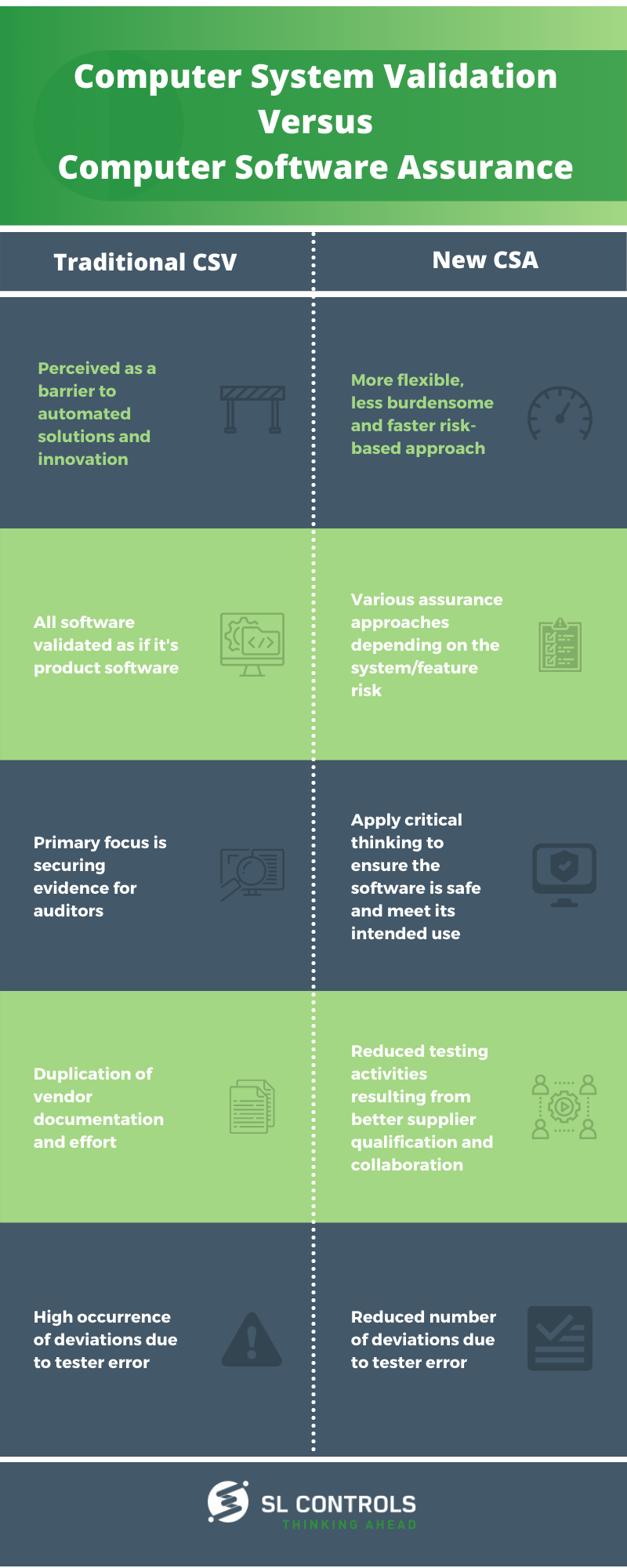
Traditional CSV
- Perceived as a barrier to automated solutions and innovation
- All software validated as if it’s product software
- The primary focus is securing evidence for auditors
- Duplication of vendor documentation and effort
- High occurrence of deviations due to tester error
New Computer Software Assurance
- A more flexible, less burdensome, and faster risk-based approach
- Various assurance approaches depending on the system/feature risk
- Apply critical thinking to ensure the software is safe and meet its intended use
- Reduced testing activities resulting from better supplier qualification and collaboration
- Reduced number of deviations due to tester error