Manufacturing in the life sciences sector is dominated by large-volume, high-speed production facilities where business models are based on economies of scale. This approach has served the sector and society very well over the past decades, especially in the developed world. Now, however, there is increasing interest, research, and innovation in a different form of production – mobile manufacturing.
Mobile manufacturing is a type of decentralized production, where manufacturing takes place closer to the point of need. It offers many potential advantages to life sciences companies as well as to patients, but there are also risks and challenges. We’ll look at all of these points in this blog.
However, it’s helpful at the outset to highlight one of the main factors driving innovation in this area – personalized medicines, treatments, and devices.
There is still a need in the healthcare sector for mass-produced pharmaceutical and medical device products, so high-speed, high-volume production facilities are here to stay. However, there is also a growing need for alternative manufacturing approaches, such as batch size of one manufacturing, to make personalized and other low-volume novel products both commercially and practically viable.
What is Mobile Manufacturing in the Life Sciences Sector?
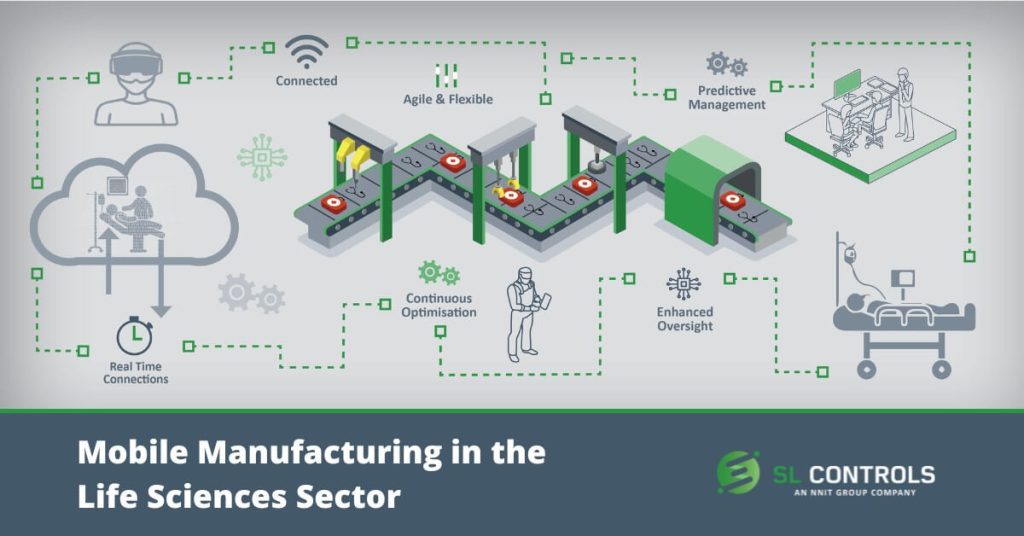
Mobile manufacturing solutions can take different forms, but they are often described as a factory-in-a-box. They often utilize continuous manufacturing processes that improve process control, enhance quality, maximize automation, improve flexibility, and increase efficiency. Mobile manufacturing solutions also often feature 3D printing and other additive manufacturing techniques.
Some of the most common characteristics of mobile manufacturing solutions include:
- Flexibility – flexibility of location as well as, often, flexibility in capacity and product, where the mobile manufacturing facility can produce different products in large or small quantities.
- Location – can be fully autonomous or installed in an existing facility, such as a hospital.
- Replicated – the unit can be replicated for multiple installations in different locations.
- Redeployment – the unit can be redeployed in a different location.
The Advantages of Mobile Manufacturing for the Life Sciences Sector
Societal and Healthcare Benefits
One of the main societal and healthcare benefits of mobile manufacturing is its potential to reduce the unequal access to medicines and treatments between developed and developing countries.
As mentioned previously, mobile manufacturing solutions also create viable production solutions for personalized medicines, including products that are difficult to manufacture using the high-volume, high-speed manufacturing model. Cell and gene therapies are a good example. The rapid manufacturing turnaround required by many cell and gene therapy products makes it highly beneficial to move production as close to the patient as possible.
Another societal and healthcare benefit of mobile manufacturing is that it enables a rapid response to patient demand, where products can be made available to patients faster than traditional manufacturing approaches.
Commercial Advantages of Mobile Manufacturing
The commercial advantages of mobile manufacturing include:
- More effective and efficient method of producing low-volume therapies and treatments.
- Potential cost-savings compared to the traditional approach of large-scale manufacturing facilities often located at a considerable distance from the point of care. This includes an enhanced ability to right-size the manufacturing process according to the product.
- Reduced capital investment risks as mobile manufacturing facilities can be built according to market demand, deferring large-scale capital expenditure until there is greater certainty of market success.
- Reduced risk of supply chain disruption where problems at one facility can have a wide impact on overall production.
Sustainability of Mobile Manufacturing
The sustainability benefits of mobile manufacturing include reductions in transportation costs to move medicines and other treatments from manufacturing facilities to the point of care. Smaller buildings/facilities also require fewer resources to build, run, and maintain, while right-sizing manufacturing based on the product can reduce the environmental impact of production.
The Commercial Factors Driving Innovation in Mobile Manufacturing
- Market size – smaller markets are more suited to mobile manufacturing and other decentralized manufacturing strategies.
- Scale of processes – small footprint processes are more suited to mobile manufacturing.
- Product maturity – where mobile manufacturing can be a more viable option for new product introductions.
- Speed to market – facility build times are faster and capital expenditure is lower with mobile manufacturing.
Practical Example of Mobile Manufacturing
This example comes from BioNTech, the German biotechnology company. In 2022, it introduced in Africa its new turnkey container-based mRNA manufacturing facilities for the production of vaccines. This mobile manufacturing solution enables the production of mRNA vaccines in bulk with fill-and-finish completed by local partners. The solution is capable of producing 50 million doses of the Pfizer-BioNTech Covid-19 vaccine a year.
Mobile Manufacturing Risks and Challenges
There are a number of risks with mobile manufacturing that must be understood and mitigated, as well as several challenges.
Cybersecurity
Mobile manufacturing facilities significantly increase the size and complexity of an organization’s attack footprint. Therefore, the development of strong cybersecurity measures and protocols must be given the same level of priority as developing the technologies that make mobile manufacturing practically and economically viable.
Physical Security
There are also physical security risks that need to be understood and mitigated, as the physical attack surface will also increase as companies develop and deploy mobile manufacturing solutions. The risks from a physical attack on a mobile manufacturing facility include everything from intellectual property theft to data privacy breaches to manipulating the manufacturing process in a way that causes harm to patients.
Regulatory and GMP Compliance Challenges
Regulations governing the life sciences sector are weighted towards traditional centralized manufacturing facilities, where volume and scale are typically the priorities. Regulations, standards, and guidance will have to continue to evolve to accommodate and make viable mobile manufacturing solutions. This includes guidance on quality, inspection, and oversight.
An example of the regulatory challenges that exist includes how to ensure the ongoing compliance status of a mobile manufacturing facility when it is relocated or replicated. Another example is how an organization’s Quality Management System can be applied to mobile manufacturing facilities.
It’s important to note that this is not a completely new area for regulators. For example, in the US, the system for people to donate blood is decentralized, with mobile blood banks inspected by the FDA every two years to ensure compliance with 21 CFR Part 606. Similar provisions exist in the EU that enable mobile blood donation facilities.
The Covid-19 pandemic also gave regulators and life science sector companies experience in completing virtual compliance inspections. Our team at SL Controls developed a virtual inspection solution that solved an immediate need during the pandemic but also has potential applications in ensuring the ongoing compliance of mobile manufacturing facilities.
Rapid Deployment and Redeployment
A key feature of successful mobile manufacturing facilities is likely to be rapid deployment and redeployment capabilities. Engineering, validation, transportation, installation, and commissioning solutions will need to be developed and refined to meet this requirement.
Skills Availability
Mass manufacturing strategies have financial advantages, but skills availability is another important consideration, especially in the life sciences sector. The availability of skills means a large percentage of production in the sector takes place within established ecosystems.
Ireland is a good example, as it has a strong life sciences sector ecosystem that includes high levels of employee skills as well as established companies that provide support services to pharmaceutical and medical device manufacturers.
With mobile manufacturing, factory-in-a-box solutions can be installed in locations with minimal skills availability. Organizations will need to develop plans to overcome this challenge by, for example, maximizing automation. Training strategies might also be part of the solution.
The Potential of Mobile Manufacturing
Mobile manufacturing is not the only way to achieve decentralized production. Furthermore, decentralized production is not going to be the best strategy in all situations. What is becoming clear, however, is that innovation in the development of medicines and other treatments is placing an increased requirement for new manufacturing solutions. Mobile manufacturing is likely to have an important role to play in bringing elements of healthcare to more people faster, more efficiently, and more economically than before.